YEARS OF CLINICAL HISTORY
Results and outcomes of Shoulder Innovations’ InSet™ glenoid technology have been studied and validated in the Journal of Shoulder and Elbow Surgery.
This study documents the long-term efficacy and safety of total shoulder replacement surgery with InSet™ glenoid implants used to reconstruct deficient, arthritic glenoid bone.
Journal of Shoulder and Elbow Surgery (2019) | https://doi.org/10.1016/j.jse.2019.01.020
Near-term results of patients treated with severe glenoid bone loss, with minimum 3, an average 4.3-year follow-up, found documented excellent radiographic and functional results using Shoulder Innovations glenoid device.
Journal of Shoulder and Elbow Surgery (2012) 21,5, 675-684
Improved implant stability of Shoulder Innovations’ InSet™ glenoid in an FEA and mechanical test model. The device demonstrated an 87% reduction in implant micromotion as compared to conventional glenoid design.
Journal of Shoulder and Elbow Surgery, (2012) 21,6, 795-803.
Minimum two-year follow-up of patients treated with InSet™ glenoids in this challenging patient population found a statistically significant increase in range of motion, reduction in pain, and high patient satisfaction.
Journal of Shoulder and Elbow Surgery (2016) 25,8, 1354–1361
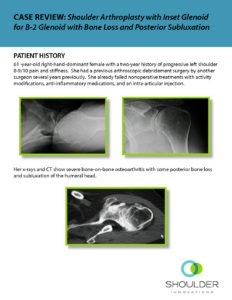
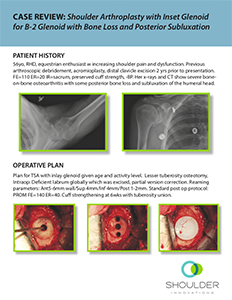
CASE REVIEW: Shoulder Arthroplasty with InSet™ Glenoid for B-2 Glenoid with Bone Loss and Posterior Subluxation
CASE REVIEW: Shoulder Arthroplasty with InSet™ Glenoid for B-2 Glenoid with Bone Loss and Posterior Subluxation





 Subscribe today and be the first to get notified on news and updates from Shoulder Innovations.
Subscribe today and be the first to get notified on news and updates from Shoulder Innovations.