Rob Ball, CEO of Shoulder Innovations provided a company update at the 2021 LSI conference.
Video Transcript
Thanks everybody, I’m Rob Ball, CEO of Shoulder Innovations and I’m just delighted to be here. I was going to try to tell a funny joke, but I realized I only have eight minutes, and so there’s no time for that. So appreciate your time.
I’m going to start with our team. We’re a shoulder arthroplasty company. We develop and manufacture and distribute shoulder arthroplasty products. This is a couple billion dollar market, very healthy economics, excellent gross margins. All those financial metrics are exactly where you want them to be, but what you really have to have is a great team.
Our team has been operating in this space literally for decades. Obviously I’m the CEO, Don, Jeff, Dave, and I have been working literally together in this space for decades. Matt’s joined us here over the past six years or so, and does a fabulous job running our finance and operations.
But what’s important about this team is our accomplishments. We’ve had a huge impact on the shoulder arthroplasty space over the past couple decades.
We all worked together at DePuy when the shoulder arthroplasty market was really created to be kind of a multi-hundred million dollar market with the Global Advantage product line.
We actually had the first reverse shoulder arthroplasty cleared in the United States. That was led by Jeff Ondrla. Jeff, Don, and Dave went on to found DVO, which is actually the source of where Tornier acquired the Simpliciti and the Cortiloc Glenoid. Those are the top selling stemless shoulder arthroplasty device in the world today. The Cortiloc Glenoid is a precursor to the number one selling glenoid implant in the world today.
I ran R&D for about eight years for Tornier. We acquired DVO, ad that’s where Tornier, got the Simpliciti implant. We also built the Ascend platform. That propelled Tornier to be the number one market share leader in the United States, actually in the world, which was then acquired by Wright Medical and further by Stryker for the largest MedTech deal ever, which I’m sure many of you know.
So we also were principals in a company called Imascap. Imascap was the software development company that created the Blueprint product. The Blueprint product was acquired by Wright Medical, which was a very important element that propelled them to number one market share position.
So we’ve had a huge impact on this marketplace and we took our position in Shoulder Innovations and I’ve been leading the company here since 2015.
Really it was based in a core technology that solves the real problem in anatomic shoulder arthroplasty, is the fact that the device that goes into the scapula comes loose.
So it’s proven in clinical literature that at five years, 20% of those devices are frankly loose, about half of them are shown the precursor of loosening in an x-ray. So that’s obviously not the type of outcome that we’re looking for for patients in shoulder arthroplasty.
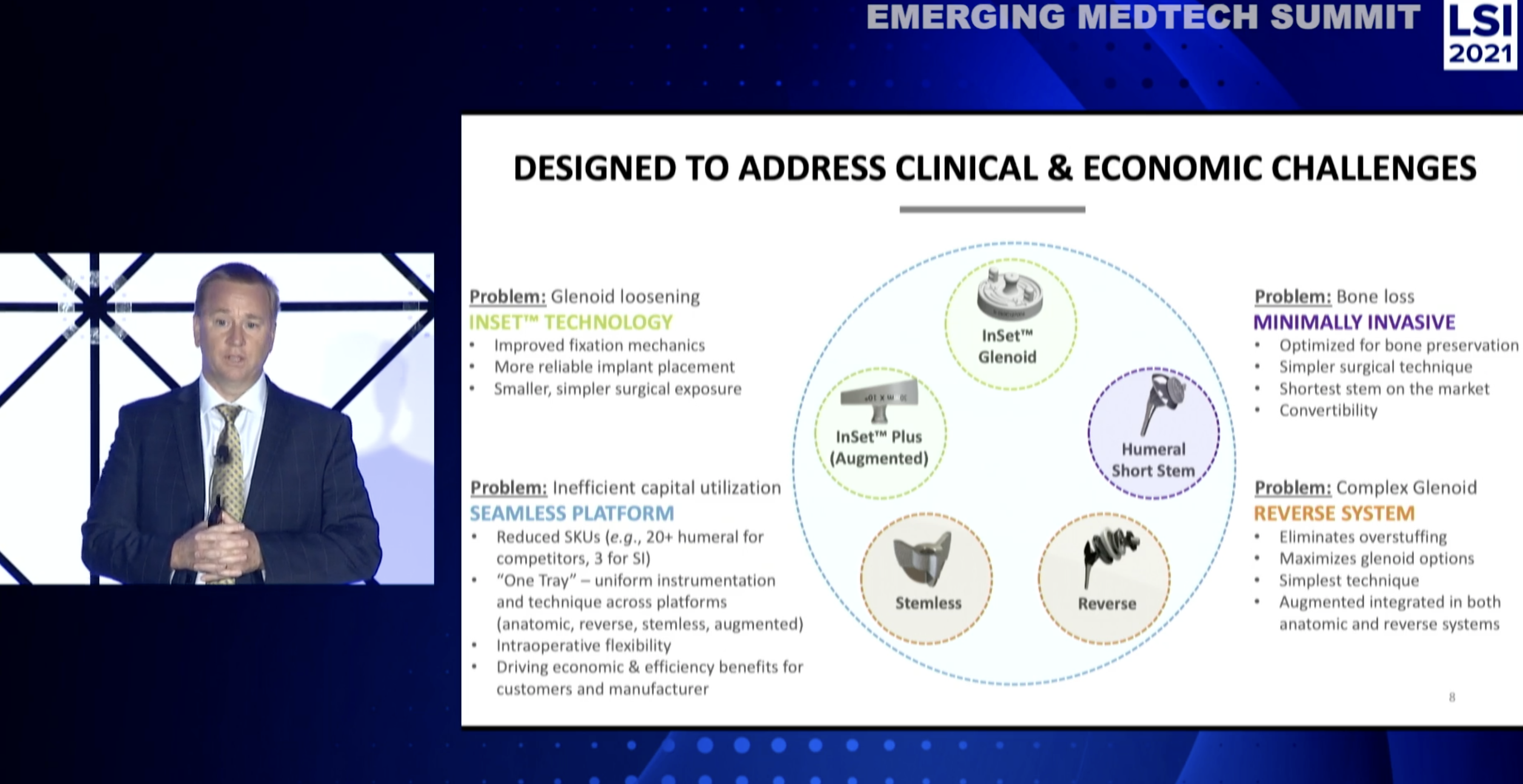
And so we developed what we call InSet™ technology that provides for excellent fixation, but kind of non-intuitively. It provides the simplest possible operation known in the glenoid arthroplasty space. And so it also provides a ton of flexibility which we’ll see later, as I chat.
And so here is the augmented, what we call the augmented form of the InSet™ technology. And you can see it creates actually a pocket of bone in that glenoid kind of space, or in that scapula. And the implant actually sits down in that pocket.
We’re the only device in the market that do that, of course, it’s kind of broadly, broadly protected with intellectual property that’s been well-supported at this point. And that provides a very rock solid fixation construct where that implant just does not move.
In fact, we’ve had clinical literature published that shows that it reduces about 90% of what we call rocking horse motion, which is indeed what causes the loosening to happen. So, you don’t have to take my word for it. We’ve got multiple peer
reviewed publications in JSES, it’s the world leading journal
for shoulder arthroplasty, up to and including by the senior author, Steve Gunther published nine year results in C type glenoids, the most challenging glenoid to treat with the glenoid
arthroplasty of this type. No loosening, no revisiting surgery, extremely happy patients.
At this point, we’ve been commercially in the marketplace with this device since 2016, thousands of implants, very, very successful, very, very happy patients and happy physicians.
And so the approach for us as a company was to start with this InSet™ technology and use that as a core platform for us to kind of begin our entrance into the marketplace.
As I mentioned, we began commercializing the InSet™ device in 2016 and we took kind of what we call a micro-commercialization model, which means for us that we had a few select territories where we invested relatively small amounts of capital in the inventory to kind of prove that device works both clinically, and we knew that it could be successful commercially.
So as we did that, we in parallel developed a whole another suite of products that represents about 85% of what happens in a shoulder arthroplasty surgeons operating room. We did that all through a scalable 13485, quality system and manufacturing supply chain.
In fact, Dave and Matt, two of my partners here, they presented this company last year at LSI. We walked out of the meeting and ended up raising just under $22 million to do what’s next, which is expand that distribution and get those product lines more broadly distributed in United States. And so we’re in the middle of doing that right now, today.
So those products look like the following. So the InSet™ glenoid and the augmented, they’re in the upper left, already solved that glenoid loosening problem. The problem in the humeral side is related to the amount of bone that gets taken.
You’ll see many, many companies currently introducing devices that take even more bone under that proximal humerus.
And so we have a very solid, very sexy fixation device on the humeral side. So we have the smallest implant, very simple technique for both the humeral and the glenoid side.
We now have the reverse system which provides convertibility, solves the major problem in reverse shoulder arthroplasty, which is biomechanics, frankly. We’re still trying to get that right in this marketplace.
And then that’s all tied together in a very seamless platform,
including our stemless device. You put all these things together and they were all developed concurrently so they all work together. They use the same surgical techniques, the same instrumentation, and so we can create a very small package.
And what’s really important is we’re able to deliver all those products, which is five or six products in the shoulder markets world into simple containers.
So you can see the blue box in the right, and the kind of silver
and green box to the left. That’s us. That’s a whole series of products packaged into a very small footprint like no one else has done before. In comparison to the rest of that stuff in the back of the pickup truck, that’s our competitor’s devices. So that’s their many products compared to our suite of products that come together as one in one package. So does that gives us a very significant competitive advantage, obviously both from a
capital outlay standpoint, but this is very costly for healthcare systems. And so what that means is we’re going to be able to lead shoulder arthroplasty into the the ambulatory surgery center space.
I think we all understand that joint replacement’s going to double here over the next 10 years. None of that growth is
happening at hospitals. It’s all going to happen at ambulatory surgery centers.
There’s no way to effectively sterilize and manage 10 trays in an ambulatory surgery center on a regular basis. And so we’ve solved that problem and we’ll be taking this into the ambulatory surgery center place.
We also just did announce yesterday, we have exclusive rights to a brand new software platform. Genesis Software Innovations has produced the PreView Shoulder software platform, AI-enabled preoperative planning solution. It’s a key driver for both
surgeons selling patients on the opportunity with shoulder arthroplasty but cementing that relationship between the commercial organization and customers, and just is an important factor that drives surgeon engagement with our company.
So that we’re excited that we now have exclusive rights to that AI platform. Clearly innovation that matters, we’ve solved some real problems. We’ve been in this space a long time. We have relationships and success in this space that’s been proven. And we know that we’re going to be able to leverage this platform to pull through significant revenues in our marketplace.
So with that, thank you for your time.
(audience applauding)