Shoulder Innovations Products
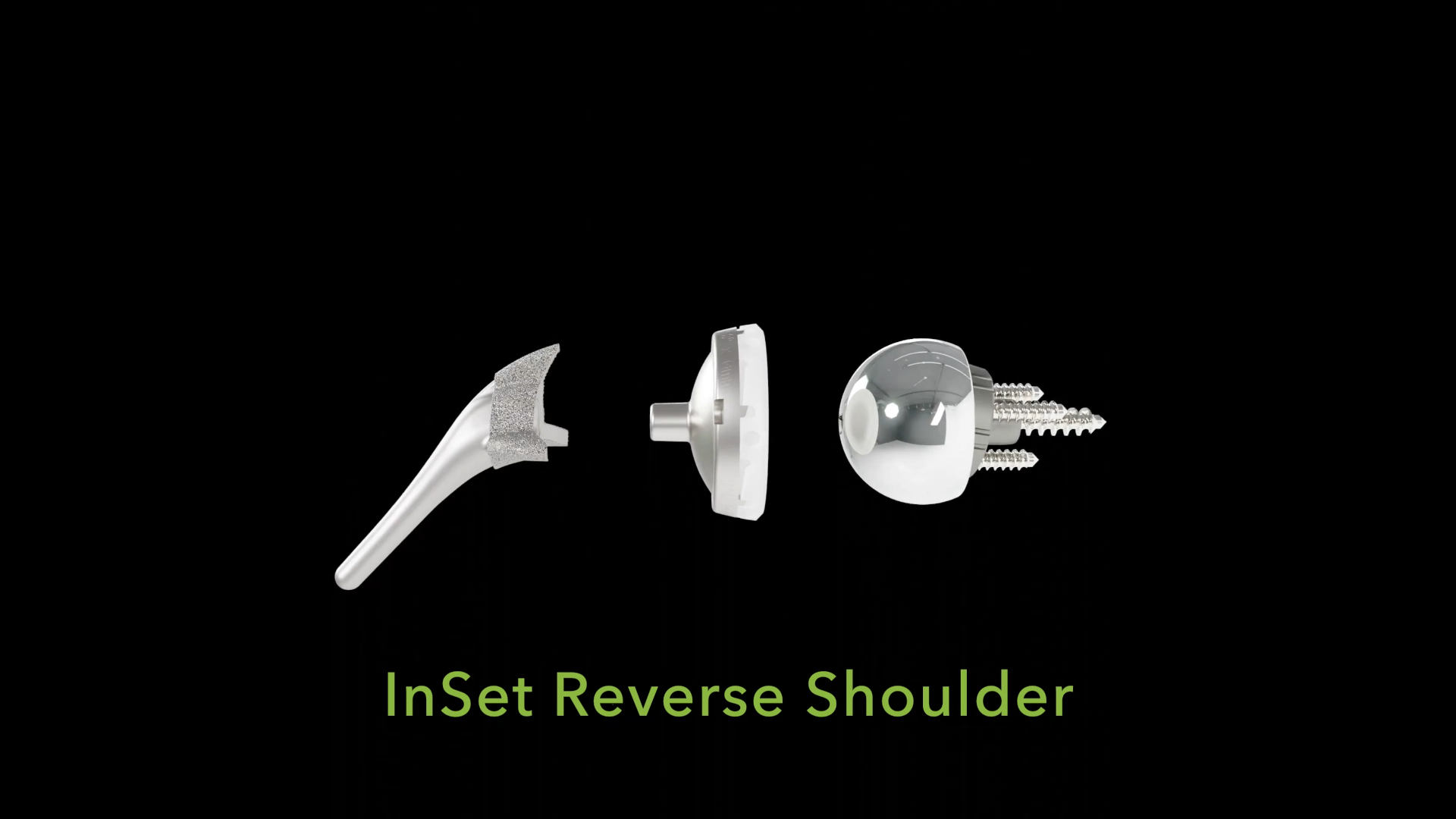
InSet™ Reverse Total Shoulder Arthroplasty

Shoulder Innovations developed the InSet™ Reverse System to optimize biomechanics by focusing on one critical question: How could we make a reverse shoulder behave more like an anatomic shoulder replacement?
By addressing this question, the InSet™ Reverse System can help surgeons maximize what remains of their patients’ native anatomy, with the goal of improving post-operative motion and overall quality of life.
The InSet™ Reverse System offers surgeons an innovative and reproducible solution for managing challenging shoulder conditions while preserving biomechanical integrity. The system can optimize rotational capacity through its lateralized glenoid, lateralized humerus construct. This thoughtful approach can position the greater tuberosity in a way that can maximize post-operative function.
Supported by a growing body of scientific evidence and real-world results, the system ensures precision in helping the surgeon restore motion and stability. By combining practical versatility with biomechanical innovation, the InSet™ Reverse System can help surgeons deliver improved outcomes for their patients.
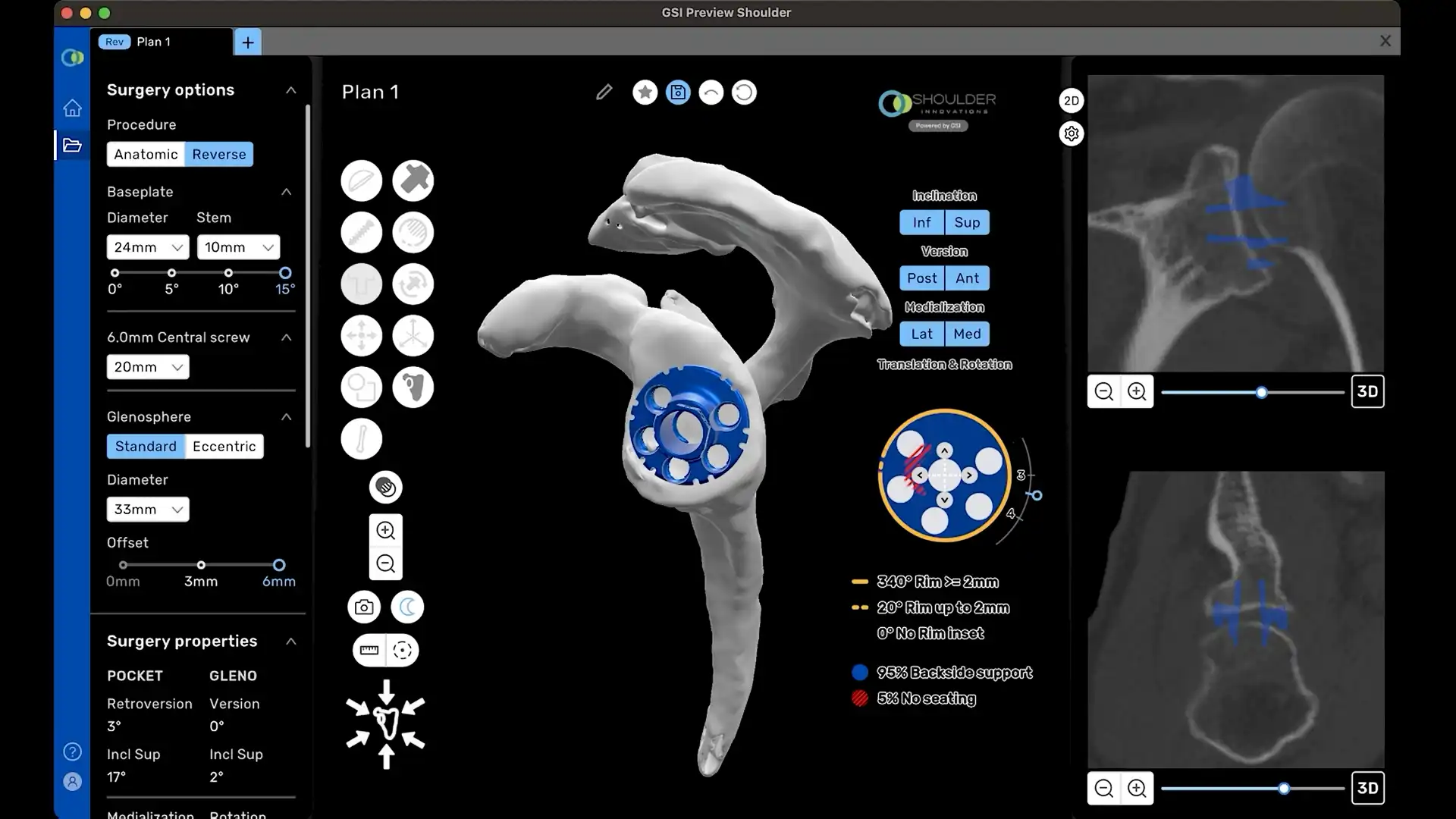
0° Neutral Baseplate
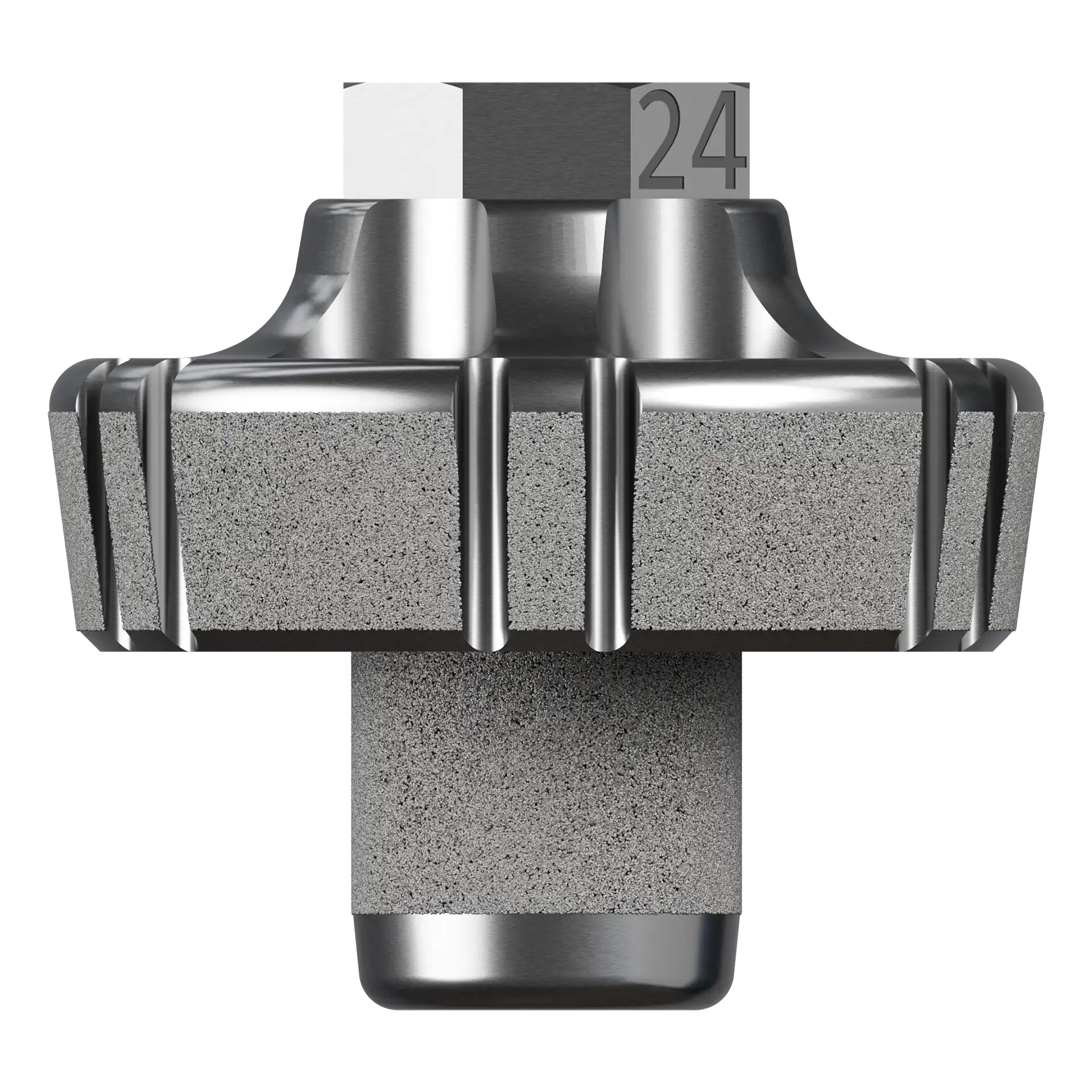
5° Augment Baseplate
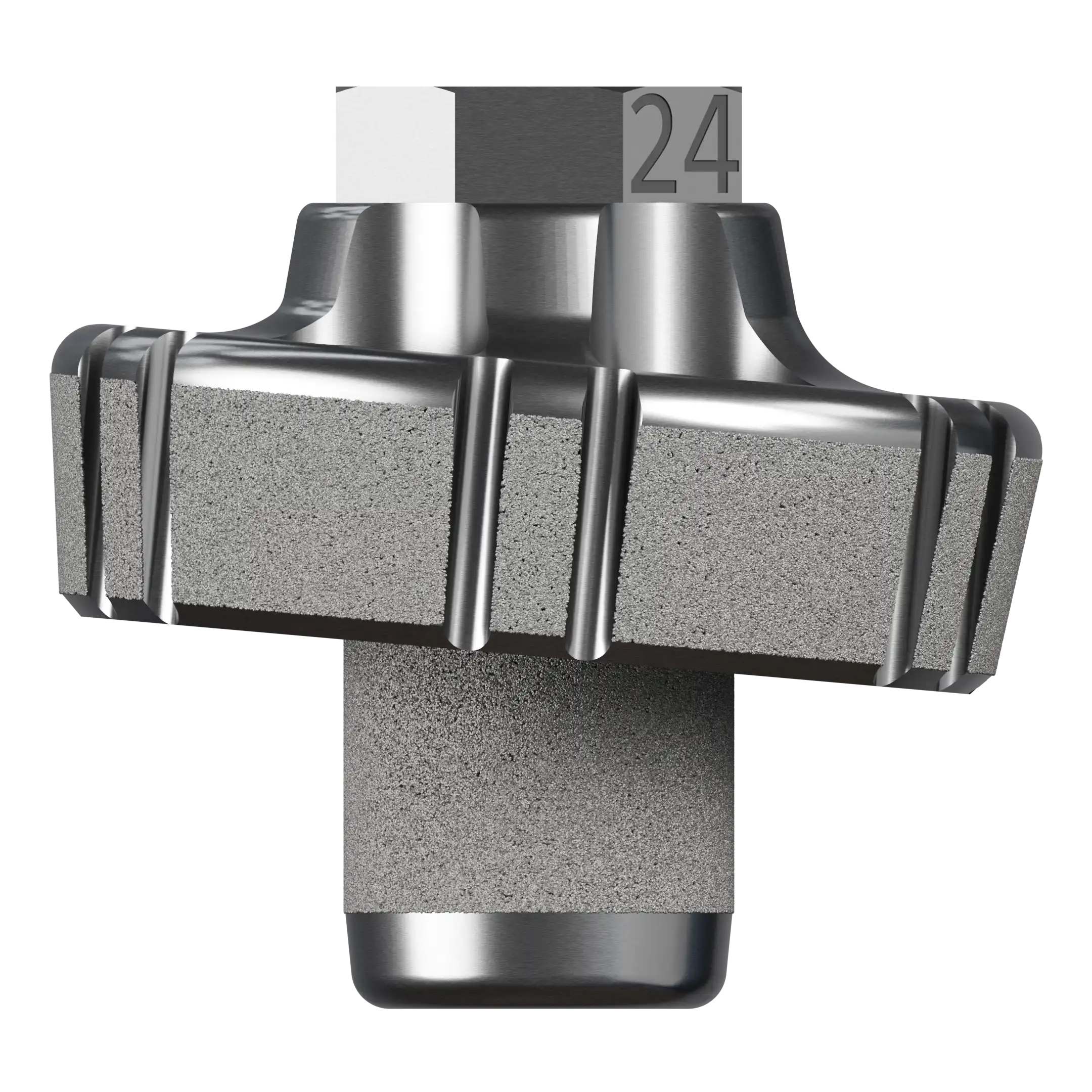
10° augment Baseplate

15° Augment Baseplate

6.0 mm Central Compression Screw
4.5 mm Variable Angle Screw/Fixed Angle Locking
Standard Glenosphere (Neutral)
Lateralized Glenosphere (+3mm & +6mm)
Short Stem
Low profile no collar on stem to maximize osteotomy loading on humeral head.
Proprietary porous coated curved fins designed to maximize rotational stability and fixation while preserving bone.
Can support a wide range of Subscapularis repair techniques.
InSet™ 95
2-fin design for rotational control.
Robust fixation aggressive proximal coating for proximal fixation along with strong metadiaphyseal fixation.
Slotted suture holes and fins.
6.0 mm Central Compression Screw
CAD RENDER
4.5 mm Variable
Angle Screw
CAD RENDER
4.5 mm Fixed
Angle Locking
CAD RENDER
Standard Glenosphere (Neutral)
CAD RENDER
Lateralized Glenosphere (+3mm & +6mm)
CAD RENDER
Set Screw &
Locking nuts
CAD RENDER
Glenosphere Locking
Bolts
CAD RENDER
Videos
0:51
0:43

16:32